DFT2#
1. Quick recap of DFT#
Correlation energy: \(E^C = E^\text{exact}-E^\text{HF}\)
Electron density: \(\rho(r)\)
Functional: \(F[\rho(r)]\), input: function, output: value
Hohenberg-Kohn Theorem:
\(E[p]\geq E_0, E[p_0]=E_0\)
Kohn-Sham auxiliary system:
\(\hat{H}_\text{aux}=\hat{F}_\text{KS}=-\frac{1}{2}\nabla^2+V_\text{eff}(\vec{r})\)
2. The Kohn-Sham Variational Equations#
Slater determinant:
\(H(\vec{x_1},...,\vec{x_N})=\frac{1}{\sqrt{N}}\begin{vmatrix}\theta_1(\vec{x_1}) & ...& \theta_N(\vec{x_1})\\\theta_N(\vec{x_N}) & ...& \theta_N(\vec{x_N})\end{vmatrix}\)
Again, the unknown term is \(E_{\mathrm{xc}}[\rho]\). Similarly as what we done in previous Hartree-Fock approximation session, we applied variational principle to make the orbitals \(\theta_i(r)\) fulfill in order to minimize this energy.
Recall that, variational principle \(\theta_i(r)\rightarrow \delta \theta_i(r), \delta \text{ is the variation factor}\)
3. Achieving Self-Consistency of the Kohn-Sham Equations#
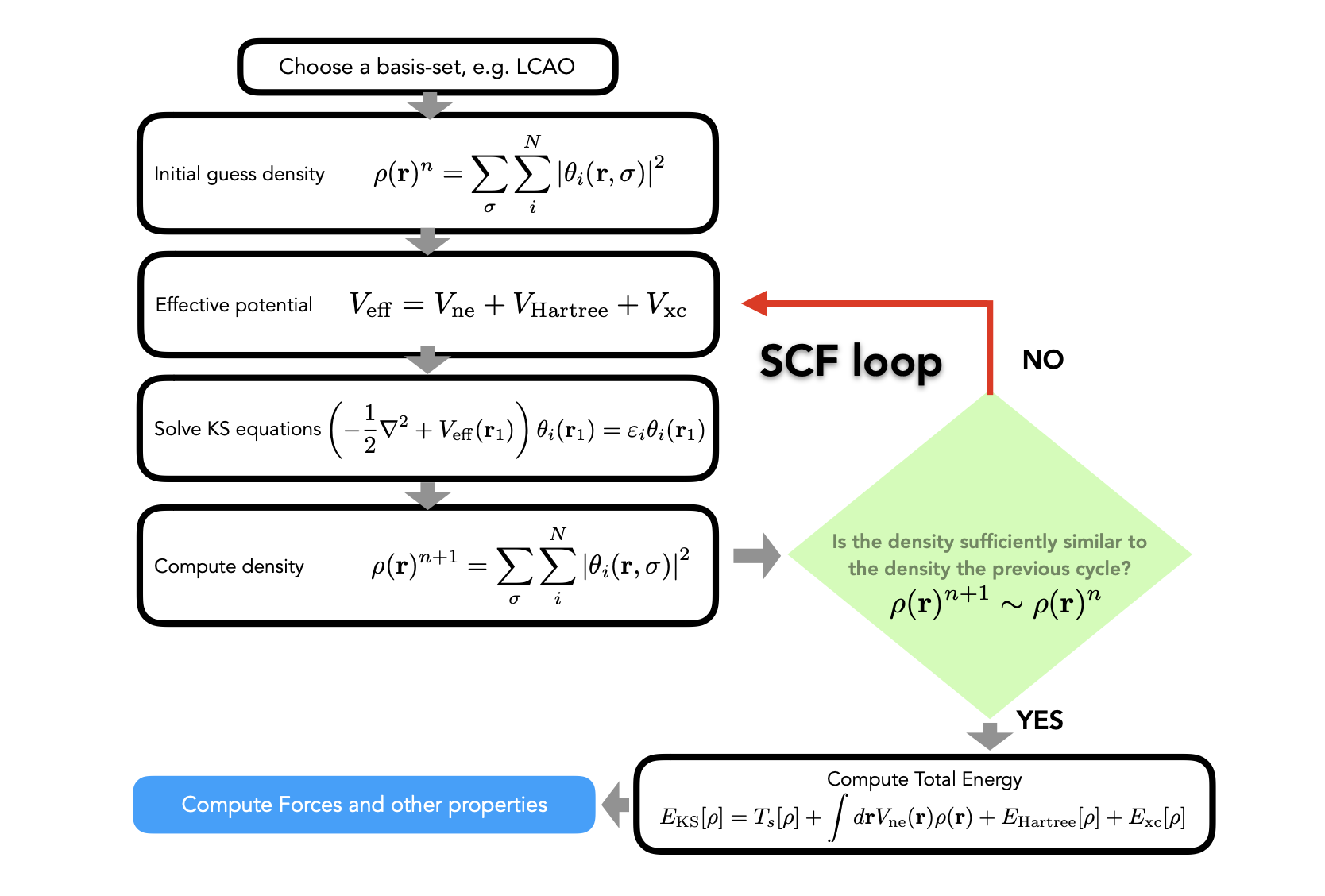
4. Finding the Unknown Exchange-Correlation Functionals#
[ \left{ \begin{aligned} &\text{1. Chemist (empirical): get }E_\text{xc}\text{ by comparing to experiments}\ &\text{e.g. heat of formation} \ &\rightarrow\text{ energy does not guarantee a better functional or }\rho \ &\text{2. Physicist: build from scratch (based on feature/constraints)}\ &\rightarrow\text{ limited known features/constraints} \end{aligned} \right. ]
Local Density Approximation (LDA)
Assumption: uniform electron gas (metallic)
where \(\epsilon_\text{xc}\) is the exchange-correlation energy per particle of a uniform electron gas of density \(\rho(r)\).
The quantity \(\epsilon_\text{xc}[\rho(r)]\) exchange and correlation contributions can be spilt as :
Meta-GGA Rung-3 functional on Jacob’s ladder of DFT. It improves over GGA by using additional semilocal information about the electron density:
\(\tau = \frac{1}{2}\sum_i|\nabla\psi_i|^2 \rightarrow\) kinetic-energy density
Kinetic energy density physical meanings:
It is large where orbitals oscillate rapidly (e.g. core, bonding), small where density is smooth.
bonding type (metallic, ionic, covalent)
iso-orbital regions (single-orbital like H atom)
weak interactions (vdW regions)